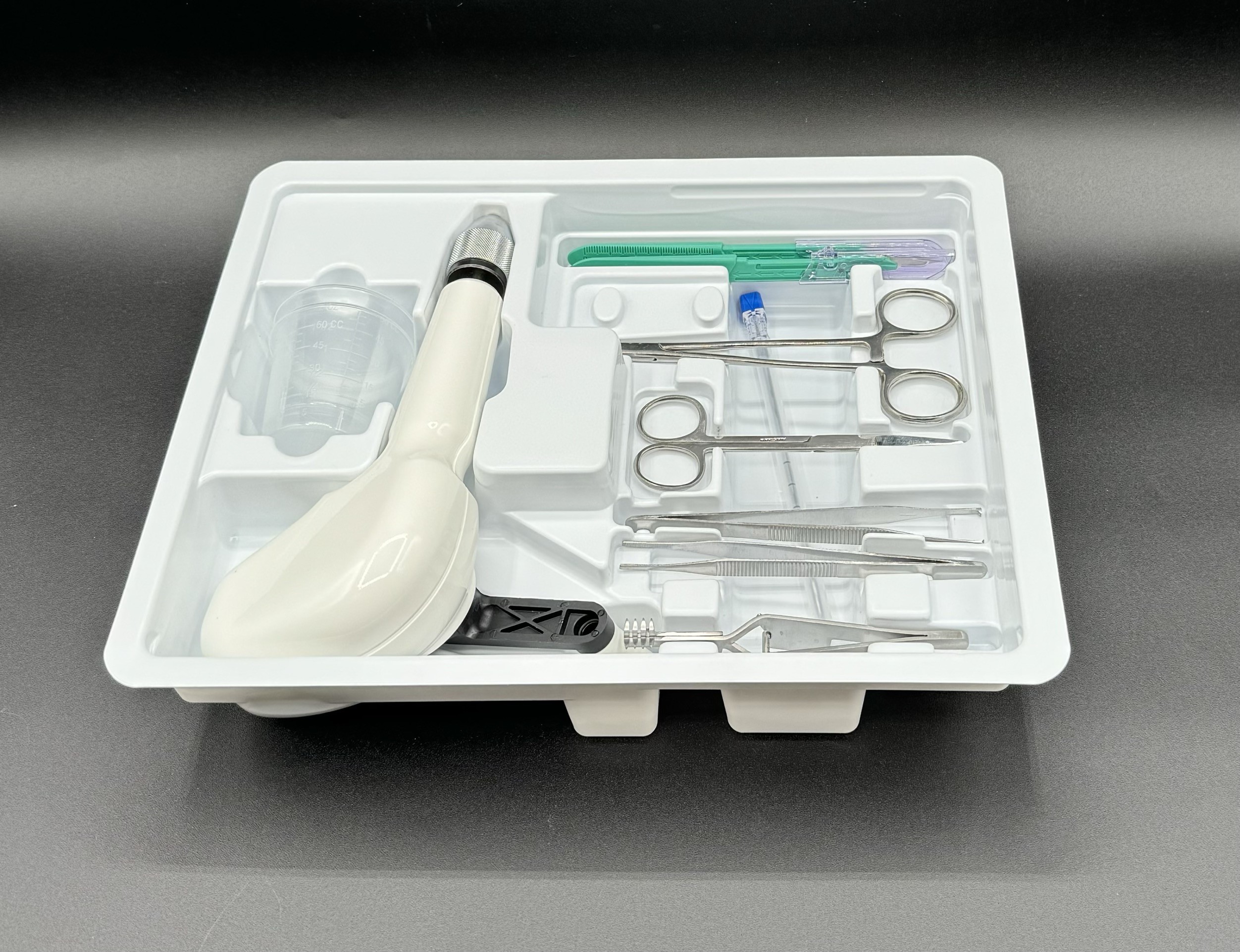
One of the biggest frustrations we hear from medical device teams is the complexity of managing multiple vendors for packaging design, validation, sterilization, and fulfillment.
Every handoff introduces delays, compliance risk, and extra cost. That’s why at Averra Packaging, we’ve built a turnkey solution designed specifically for the medical device industry.
From packaging design and ISO 13485–aligned validation support to biocompatibility studies, sterilization, and fulfillment — we help streamline the entire process so engineering and quality teams can focus on what matters most: getting life-saving devices to patients faster.
If your team is facing packaging challenges or validation bottlenecks, let’s connect. We’d love to hear how you’re currently managing the process and share a few ways we’ve helped other device companies reduce lead times by 20–30%.